|
SICKLE-CELL DISEASE AND RHABDOMYOLYSIS
by Katie
Olson, undergraduate student University
of Florida
Although exercise is highly beneficial
for virtually all people, when taken
to an extreme, especially in individuals
with particular risk factors, it can
become dangerous. A well-known but often
underestimated risk factor is sickle-cell
anemia, sometimes called sickle-cell
trait when an individual carries
only one sickle-cell allele,
which is exacerbated by exercise and
can lead to death. A less prevalent
and thus less recognized outcome of
excessively strenuous exercise is rhabdomyolysis,
a severe condition involving the breakdown
of muscle tissue and eventually renal
failure resulting in death.
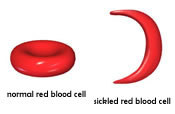 Sickle-cell
disease is a recessive, genetically-inherited
disease that causes an individual's
red blood cells (RBCs) to form abnormally
(Kark, 2000).
Instead of forming smooth, round discs
that pass easily through small capillaries,
an individual with sickle-cell disease
has sticky red blood cells shaped like
sickles or crescent moons. These red
blood cells stick to one another and
the walls of capillaries, blocking the
flow of blood, and therefore oxygen
and nutrients, to tissues. Reduced blood
flow to an organ causes organ damage,
and in the case of sickle-cell disease
the spleen is most often affected (Tsaras,
Owusu-Ansah, Boateng, & Amoateng-Adjepong,
2009). Because the spleen aids
in immunity, individuals with sickle-cell
disease are at a higher risk of contracting
infectious diseases. Also, sickle-cell
red blood cells die more rapidly than
normal red blood cells, resulting in
a continuously low RBC count, or anemia.
Depending on the individual, this anemia
may be hidden or it may cause chronic
fatigue, dizziness, cold extremities,
headache, or shortness of breath (USDHHS,
2008). Sickle-cell
disease is a recessive, genetically-inherited
disease that causes an individual's
red blood cells (RBCs) to form abnormally
(Kark, 2000).
Instead of forming smooth, round discs
that pass easily through small capillaries,
an individual with sickle-cell disease
has sticky red blood cells shaped like
sickles or crescent moons. These red
blood cells stick to one another and
the walls of capillaries, blocking the
flow of blood, and therefore oxygen
and nutrients, to tissues. Reduced blood
flow to an organ causes organ damage,
and in the case of sickle-cell disease
the spleen is most often affected (Tsaras,
Owusu-Ansah, Boateng, & Amoateng-Adjepong,
2009). Because the spleen aids
in immunity, individuals with sickle-cell
disease are at a higher risk of contracting
infectious diseases. Also, sickle-cell
red blood cells die more rapidly than
normal red blood cells, resulting in
a continuously low RBC count, or anemia.
Depending on the individual, this anemia
may be hidden or it may cause chronic
fatigue, dizziness, cold extremities,
headache, or shortness of breath (USDHHS,
2008).
Even if an individual's sickle-cell
disease is not problematic on a daily
basis, it could escalate to a sickle-cell
crisis, which is sometimes life-threatening.
A sickle-cell crisis usually arises
from the combination of several risk
factors, all of which put added stress
on the body. For instance, dehydration
makes the blood more viscous,
its solvents more concentrated, and
its vessels to shrink, all of which
inhibit the flow of blood and increases
the likelihood of the sickle cells aggregating
(Kark, 2000).
Strenuous exercise or labor also places
stress on the body by increasing oxygen
demands and causing dehydration (Scheinin
& Welti, 2009). Life stresses,
extreme temperatures, high altitudes
and vasoconstricting
drugs also increase the risk of a sickle-cell
crisis by decreasing blood vessel diameter,
increases the likelihood of sickle cells
aggregating and causing ischemia
(Kark, 2000).
The first sign of a sickle-cell
crisis is extreme pain, usually
after exposure to any of the above risk
factors. If left untreated, a sickle-cell
crisis can lead to blindness, organ
damage, a stroke, or acute
chest syndrome, a blockage of blood
vessels in the lungs that causes chest
pain and difficulty breathing (Tsaras
et al., 2009). Immediate treatment
includes administration of intravenous
fluids and analgesics
for the sickle-cell crisis, as well
as specific treatment for any of the
many complications (Kark,
2000).
However, prevention is always preferable
to treatment, and although there is
no cure for sickle-cell disease, there
are many ways to limit its effects.
Simple precautions, such as staying
hydrated and taking supplements for
micronutrients, like folic acid involved
in the formation of red blood cells,
are highly successful in preventing
sickle-cell crises (Kark,
2000). More long-term treatments,
such as bone marrow transplants, are
also useful for preventing complications
of sickle-cell disease (USDHHS,
2008). Also, perhaps the most
critical component of prevention is
screening, because an individual who
collapses and is known to have sickle-cell
trait is more likely to be properly
diagnosed than an individual whose sickle-cell
trait was previously undetected.
 Rhabdomyolysis
is another condition that can result
from extreme exercise, often coupled
with poor hydration and hyperthermia,
although it also has many other causes
both physical and non-physical. Muscular
trauma is the most common cause of rhabdomyolysis,
and can result from something obvious
like a car accident or physical abuse,
but also can result from long periods
of confinement in a particular position,
often due to a coma, stroke, or drunken
stupor which limits blood flow to the
area and causes muscle cell death (Huerta-Alardin,
Varon, & Marik, 2004). Rhabdomyolysis
is another condition that can result
from extreme exercise, often coupled
with poor hydration and hyperthermia,
although it also has many other causes
both physical and non-physical. Muscular
trauma is the most common cause of rhabdomyolysis,
and can result from something obvious
like a car accident or physical abuse,
but also can result from long periods
of confinement in a particular position,
often due to a coma, stroke, or drunken
stupor which limits blood flow to the
area and causes muscle cell death (Huerta-Alardin,
Varon, & Marik, 2004).
Any other pre-existing condition that
limits blood supply, such as a thrombosis
or embolism,
also increases the risk of developing
rhabdomyolysis. Other risk factors are
primarily metabolic
enzyme deficiencies, such as carnitine
deficiency, CPT
deficiency, and phosphofructokinase
deficiency (Malik,
1998). Without these enzymes,
the muscle does not receive an adequate
supply of ATP,
particularly during times of stress
such as intense exercise. All of these
risk factors are heightened when a person
has insufficient glucose
in the bloodstream, electrolyte
imbalances, and insufficient hydration
(Kahn, 2009).
Rhabdomyolysis
commences when any of the above factors
cause muscle fibers to rupture, spilling
their contents into the surrounding
tissue and eventually into the bloodstream.
Some of the released chemicals include
creatine
phosphokinase (CPK), potassium,
phosphate,
lactic
and uric
acid, and myoglobin,
all of which cause devastating effects
floating freely throughout the body
(Malik, 1998).
For instance, the exiting phosphate
causes calcium to pour into the muscles,
resulting in further stimulation and
contraction of the already distressed
muscles. The escaping lactic and uric
acids lower the pH of the blood and
consequently the urine, leading to many
of the problems associated with acidosis
and aciduria
(Malik, 1998).
The potassium leaking into the bloodstream
naturally results in hyperkalemia,
which is a life-threatening condition
in and of itself. The presence of myoglobin
in the blood stream is also one of the
factors leading to the renal
failure associated with rhabdomyolysis
as the myoglobin becomes lodged in tubules
in the kidney and causes oxidation because
of its high iron content (Kahn,
2009). As water rushes to the
damaged muscle in the form of swelling,
blood volume decreases, lowering blood
pressure and raising overall blood solute
concentrations contributing to the stress
on the kidney (Malik,
1998). This decrease in kidney
functionality, coupled with the increased
workload placed on the kidney by higher
uric acid concentrations in the blood
and electrolyte imbalance, all lead
to renal failure if left untreated.
These many physiological imbalances
and problems lead to some visible signs
and symptoms, such as extreme muscle
pain and swelling, weakness, and dark
or reddish urine due to myoglobin in
the urine (Kahn,
2009). Testing will also reveal
elevated levels of CPK
in the blood, which can be attributed
to rhabdomyolysis rather than a myocardial
infarction when the other symptoms
are considered as well (Huerta-Alardin
et al., 2004). Tests revealing
hyperkalemia,
hypocalcemia,
and myoglobin
in the urine would also point toward
rhabdomyolysis,
as well as a recent history of suffering
some physical trauma.
Once a diagnosis of rhabdomyolysis
has been achieved, the primary goal
is to stabilize the patient and prevent
further renal damage (Huerta-Alardin
et al., 2004). The patient should
receive large quantities of intravenous
fluids to dilute the high blood solute
concentrations and compensate for the
fluid lost to the swollen muscle tissue.
Certain drugs that promote urine formation
should also be administered to stimulate
recovered kidney function and secrete
the superfluous
solvents
in the blood (Malik,
1998). Any other conditions accompanying
the rhabdomyolysis, such as shock, should
be treated accordingly, and often the
patient is fully healed and ready to
go home after a few weeks of bed rest.
The key to such a successful outcome
is early detection, which in the case
of exercise or sports would require
a coach to take a player's complaint
of pain and fatigue seriously.
Because a sickle-cell crisis and
rhabdomyolysis can both result from
excessively strenuous exercise, it is
critical for all coaches and trainers
in sports and exercise to be informed
of these diseases so they know what
to look for. Athletes also need to know
of the risk factors and keys for prevention,
in addition to being screened for pre-existing
conditions. Almost every year a football
player, often with sickle-cell trait,
collapses and dies the first day of
fall practice, his body overwhelmed
by high heat, dehydration, and the stress
of a workout it has not endured in several
months.
Even worse, five out of ten deaths
in Division I-A football are caused
by sickle-cell trait, a condition prevalent
in eight percent of U.S. African-Americans
(Dodd, 2009).
Despite such tragedies, only two-thirds
of Division I-A schools screen for sickle-cell
trait, a test that only costs ten dollars
but could save so many lives (Dodd,
2009). Additionally, coaches
and trainers need to accept the newest
research, which shows that not only
individuals with full-blown sickle-cell
disease, but also with sickle-cell trait,
are at risk for exercise sickling.
Only then can people finally stop dying
of a disorder that is completely preventable.
Coaches and trainers need to take
similar steps for the prevention or
successful treatment of rhabdomyolysis.
Awareness is the first key: while many
people hold misconceptions of sickle-cell
trait, viewing it as less dangerous
than it actually is, most people are
entirely unaware of rhabdomyolysis.
People in the sports world must learn
to differentiate rhabdomyolysis from
heatstroke and ailments with similar
symptoms so that proper treatment can
be initiated.
Also, athletes should be screened
for pre-existing risk factors, such
as metabolic enzyme deficiencies. Once
proper screening and awareness have
been achieved, even individuals with
pre-existing conditions, like sickle-cell
disease, can reap the multitudinous
benefits of exercise in a safe environment
(Al-Rimawi &
Jallad, 2008).
references
|



